The production of ammonia N2( g ) + 3 H2( g ) 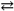 2 NH3( g ) represents a(n) :
2 NH3( g ) represents a(n) :
A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium
Correct Answer:
Verified
Q5: If NaCl is added to a saturated
Q6: Which "K" value indicates a reaction most
Q7: Which of following species is a Brønsted-Lowry
Q8: If a reaction is in a state
Q9: The reaction: 2 A( s ) +
Q11: The higher the Ksp of the salt,
Q12: Unlike traditional concrete, engineered cementitious composites (ECC)
Q13: Products appear in the numerator of the
Q14: Cememt structures with many small cracks are
Q15: In a dynamic equilibrium:
A) the rate of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents