What is the correct Lewis structure for SF4?
A) 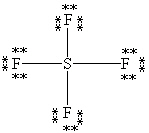
B) 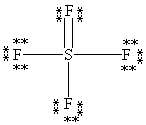
C) 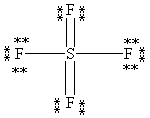
D) 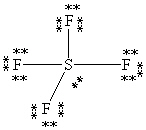
E) 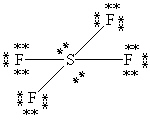
Correct Answer:
Verified
Q97: Pick the correct Lewis structure shown below.
A)
Q98: The difference in electronegativity between a boron
Q99: A possible Lewis structure for BeCl₂ is
Q100: Possible resonance forms for ClO3 - are
Q101: Given the average bond energies listed below,
Q103: Which molecule listed below has a total
Q104: Given the average bond energies listed below,
Q105: Pick the correct Lewis structure.
A) 
Q106: Given the three molecules below, which choice
Q107: In which of the following molecules does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents