Which system at equilibrium shifts right upon heating?
I. PCl 3 (g) + Cl₂ (g) 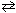 PCl5 (g) + 111 kJ
PCl5 (g) + 111 kJ
II. N₂(g) + 3 H₂ (g) 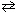 2 NH₃(g) (exothermic)
2 NH₃(g) (exothermic)
III. N₂(g) + O₂(g) 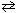 2 NO ( D H 0)
2 NO ( D H 0)
A) I only
B) II only
C) III only
D) I and II
E) I and III
Correct Answer:
Verified
Q60: Consider the Haber process for preparing ammonia
Q61: At 125 C, K p = 5.2*10-
Q62: Which of the following reactions is product
Q63: Which system at equilibrium shifts right ?
I.
Q64: For the high temperature equilibrium system, 2
Q66: Consider the following endothermic reaction at equilibrium:
2
Q67: The only factor that causes a change
Q68: Which of the following reactions is product
Q69: At 500 C, K p = 4.9*10-
Q70: What is the effect upon addition of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents