The heat of vaporization of water is 540 cal/g. What mass of water at 100.0 °C can be vaporized by the addition of 55.0 kcal of heat?
A) 0.19 g
B) 55 g
C) 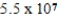 g
g
D) 102 g
Correct Answer:
Verified
Q22: When alcohol is mixed with water which
Q28: Which of the following phase changes does
Q29: Convert 0.662 atm to millibars.
A) 377 millibar
B)
Q30: Which of the following molecules cannot engage
Q34: Which of the following is the strongest
Q36: On a stove we have two pots
Q37: Which of the following molecules is most
Q38: Which of the following does not affect
Q44: Which of the following correctly arranges the
Q104: At high altitudes atmospheric pressure is lower
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents