If we know the amount of light that is absorbed in a solution, we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  , where
, where 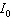 is the intensity of the incident light and
is the intensity of the incident light and 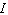 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 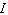 is 45% of
is 45% of 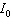 ?
?
A) 952 mol/L
B) -3,667 mol/L
C) 2,928 mol/L
D) 2,192 mol/L
E) 1,272 mol/L
Correct Answer:
Verified
Q22: If the hydrogen ion concentration of a
Q23: Which one of the following is the
Q24: Evaluate the expression Q25: The initial size of a population is Q26: Which one of the following is the Q30: Which one of the following graphs represents![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents