Phosphine, PH3, is structurally much like ammonia. Which of the following is the best Lewis structure of phosphine?
A) 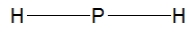
B) 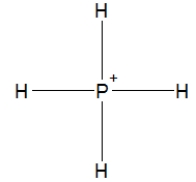
C) 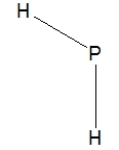
D) 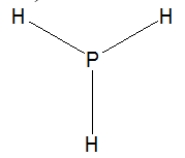
Correct Answer:
Verified
Q25: What is the correct Lewis structure for
Q26: The simplest covalently bonded molecule is hydrogen.
Q27: Which is the best Lewis structure for
Q28: Which is the best representation of a
Q29: How is ethane best represented by a
Q31: Hydrogen sulfide is considered the "rotten egg"
Q32: What is wrong with the Lewis structure:
Q33: What does the double line, which looks
Q34: Which of the following choices is the
Q35: Propane, C3H8, is best represented by which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents