Boron trifluoride, BF3, is a corrosive material with a Lewis structure that shows boron to be two electrons short of a full octet. Which is the correct structure for BF3?
A) 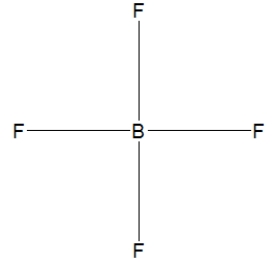
B) 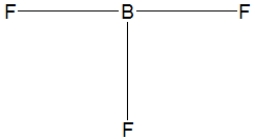
C) 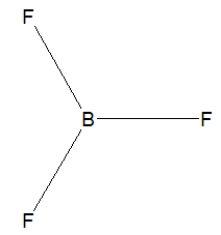
D) None of the above.
Correct Answer:
Verified
Q31: Hydrogen sulfide is considered the "rotten egg"
Q32: What is wrong with the Lewis structure:
Q33: What does the double line, which looks
Q34: Which of the following choices is the
Q35: Propane, C3H8, is best represented by which
Q37: What is the correct Lewis structure for
Q38: Bromine in the gaseous state is Br2
Q39: What is wrong with this Lewis structure
Q40: Draw for yourself the Lewis structure of
Q41: Which is the name of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents