Use the following figure to answer the question below.
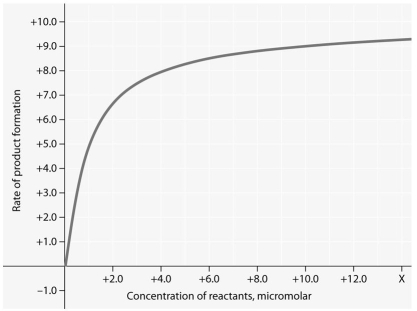
Rate of an enzyme-catalyzed reaction as a function of varying reactant
concentration,with the concentration of enzyme constant.
-When is a reaction at chemical equilibrium?
A) when the reactants are at the same concentration as the products
B) when the forward and backward reactions occur at the same rate
C) when there is no reactant remaining
D) when there is no product remaining
E) A reaction never reaches chemical equilibrium.
Correct Answer:
Verified
Q57: Q62: Succinate dehydrogenase catalyzes the conversion of succinate Q63: Succinate dehydrogenase catalyzes the conversion of succinate Q65: The following questions are based on the Q70: The following questions are based on the Q72: The following questions are based on the Q78: The following questions are based on the Q81: An organic molecule is required for the Q82: A molecule binds between two subunits and Q83: Use the following information to answer the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents