Arrange these compounds in order of increasing boiling point (lowest boiling point first) .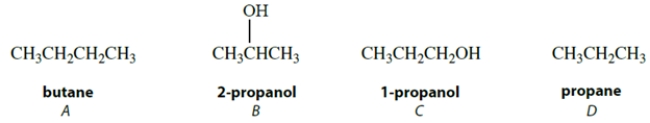
A) A < B < C < D
B) B < C < D < A
C) D < A < C < B
D) D < A < B < C
Correct Answer:
Verified
Q4: When DMSO dissolves potassium chloride (K+ Cl−),
Q5: When two hydrocarbon molecules (such as hexane),
Q6: Dissolving hexane in water has
A) a large
Q7: The boiling point of pentane is 36
Q8: Sodium chloride, an ionic compound, should
Q10: In each case, identify the better solvent
Q11: By circling three items below each
Q12: Which compound would form the strongest complex
Q13: In which solvent should NaCl (an ionic
Q14: Until the FDA rescinded the permit for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents