The equilibrium constant, Kc, was found to be 1.2 × 103 at 25°C for the reaction,2X(s) + Cu2+(aq)  2X+(aq) + Cu(s) Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X?
2X+(aq) + Cu(s) Using the following reduction potential for copper, what is the reduction potential for the other half reaction involving the substance X? 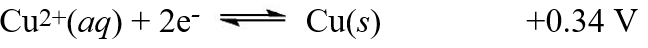
A) -0.16 V
B) 0.091 V
C) 0.52 V
D) 0.18 V
E) -0.25 V
Correct Answer:
Verified
Q43: A galvanic cell is composed of
Q44: A galvanic cell is composed of
Q45: Using the standard reduction potentials
Q46: Using these metal ion/metal standard reduction
Q47: Using the reduction potentials given, calculate the
Q49: Using the reduction potentials given, calculate the
Q50: The equilibrium constant, Kc, was found to
Q51: A galvanic cell is composed of these
Q52: A galvanic cell is composed of these
Q53: A galvanic cell is composed of these
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents