The normal melting point of carbon dioxide is -78°C. Predict the signs of H, S, and G for the process in which solid carbon dioxide sublimes at -50°C and 1 atm: CO2(s) .CO2(g) 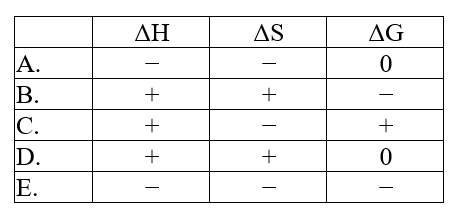
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q24: Which set below has the species listed
Q25: For a certain chemical reaction,
Q26: For a certain chemical reaction,
Q27: The requirement for a spontaneous chemical
Q28: The normal melting point of benzoic
Q30: The normal melting point of naphthalene
Q31: Which property associated with a chemical
Q32: A negative sign for
Q33: For the reaction 2NO(g)+ O2(g)
Q34: According to the second law of thermodynamics,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents