Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants: 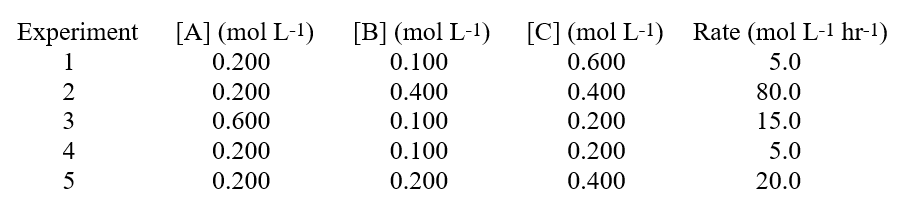 Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.
A) the reaction is zero order with respect to B.
B) the reaction is first order with respect to B.
C) the reaction order for B cannot be determined.
D) the reaction is second order with respect to B.
E) the reaction order for B is minus one.
Correct Answer:
Verified
Q29: For the reaction, 2M + 2N
Q30: For the reaction, A + 2B
Q31: For the reaction, A + 2B
Q32: The reaction, 2A2X4(g)
Q33: The data below were obtained in a
Q35: Given these data in a study on
Q36: Given these data in a study on
Q37: What is the order of the
Q38: What is the order of the
Q39: The data shown below was determined
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents