A solution of ethylene glycol (C2H6O2) in water is 3.981 molar and has a density of 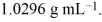 Calculate the percent, by weight, of ethylene glycol in the solution.
Calculate the percent, by weight, of ethylene glycol in the solution.
A) 3.867%
B) 4.099%
C) 15.14%
D) 24.00%
E) 25.45%
Correct Answer:
Verified
Q25: A solution is prepared by mixing 0.3355
Q26: An aqueous solution is prepared by mixing
Q27: An aqueous solution of nitric acid has
Q28: Which is a concentration unit whose value
Q29: Consider a 0.900 M Al(NO3)3 solution. This
Q31: A solution of sodium nitrate, NaNO3, in
Q32: An aqueous solution of ethanol, C2H5OH, is
Q33: An aqueous solution of glycerol, C3H8O3, is
Q34: A solution is prepared by mixing 0.3355
Q35: An aqueous solution of glycerol, C3H8O3, in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents