Using the standard enthalpies of formation, ΔH°f: 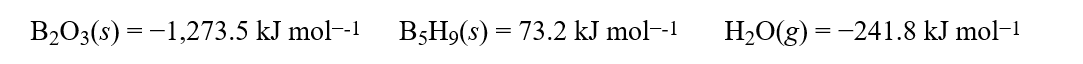 calculate how much energy would be given off when 104.4 g of B5H9(s)burns in an oxygen environment to produce B2O3(s)and H2O(g).Hint: Write out the combustion equation and balance it.
calculate how much energy would be given off when 104.4 g of B5H9(s)burns in an oxygen environment to produce B2O3(s)and H2O(g).Hint: Write out the combustion equation and balance it.
Correct Answer:
Verified
Q119: Complete combustion of hydrocarbons or compounds with
Q120: Complete combustion of hydrocarbons or compounds with
Q121: Using the standard enthalpies of formation, ΔH°f:
Q122: Using the standard enthalpies of formation, ΔH°f:
NO(g)=
Q123: Using the standard enthalpies of formation, ΔH°f:
CO(g)=
Q125: The statement, "the total energy of the
Q126: The potential energy of an object can
Q127: Heat is energy that is transferred between
Q128: Temperature is a measure of the average
Q129: A property, like energy, that depends only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents