In the course of determination of a chemical formula, a student obtained the following mole ratios: 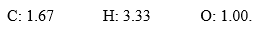 The empirical formula for the compound is ________.
The empirical formula for the compound is ________.
Correct Answer:
Verified
Q135: The percent, by weight, of oxygen in
Q136: How many grams of oxygen are present
Q137: In the course of determination of a
Q138: In the course of determination of a
Q139: In the course of determination of a
Q141: A compound contains 46.46% lithium by mass
Q142: The percent composition by mass of a
Q143: A 1.375 g sample of mannitol, a
Q144: The empirical formula of a compound composed
Q145: When the equation is balanced, Al2O3 +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents