Lactic acid (line structure below) is an intermediate in the metabolism of glucose.(C = 75.291 J/mol.K for H2O; q = Ccal T) 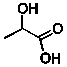 When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)
When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 2.71°C
B) 26.15°C
C) 25.11°C
D) 27.23°C
E) 28.34°C
Correct Answer:
Verified
Q16: The combustion of an organic compound
Q17: A constant pressure "coffee-cup" calorimeter is
Q18: At 11:30 pm at a large
Q19: A constant volume calorimeter's temperature increases
Q20: How much energy is required to
Q22: How much energy in kJ must
Q23: How much energy must be removed
Q24: A 1.72 g sample of the
Q25: A piece of cauliflower was combusted
Q26: What is the work (per mole
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents