Multiple Choice
Solve the problem.
-According to the Gas Kinetic Theory, the average speed of a gas particle is given by 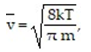 where
where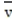 is the speed in m/s, k is the constant 1.38 × 10-23 , T is the temperature of the gas in Kelvin, and m is the mass of the gas particle in kg. What is the average speed of an oxygen molecule with a mass of
is the speed in m/s, k is the constant 1.38 × 10-23 , T is the temperature of the gas in Kelvin, and m is the mass of the gas particle in kg. What is the average speed of an oxygen molecule with a mass of
5.314 X 10-26 KG at a temperature of 500 K?
A) 1020 m/s
B) 575 m/s
C) 330,700 m/s
D) 174 m/s
Correct Answer:
Verified
Related Questions

