Calculate the value of the Ksp for CdS at 25 °C from the following data.
CdS + 2 e- 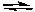 Cd + S2- E° = -1.21 V
Cd + S2- E° = -1.21 V
Cd2+ + 2 e- 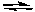 Cd E° = -0.40 V
Cd E° = -0.40 V
A) 3 x 10-55
B) 4 x 10-28
C) 2 x 10-14
D) 3 x 1027
E) 6 x 10
Correct Answer:
Verified
Q37: Which of the following reagents should react
Q38: Which of the following pairs of ions
Q39: Cobalt(III) oxide reacts with hydrogen gas
Q40: The equilibrium constant at 298 K
Q41: The equilibrium constant at 25 oC
Q43: There are two half-reactions for the reduction
Q44: The half-reaction reduction potentials for the mercury(I)
Q45: Calculate the complex dissociation equilibrium constant (Kd)
Q46: Calculate the weight of sodium metal that
Q47: What is the ratio of the weight
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents