refer to the following incomplete, unbalanced equation
Cr2O72-(aq) + NO(g)
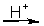 Cr3+(aq) + NO3-(aq)
Cr3+(aq) + NO3-(aq)
-How many electrons are in the half-reaction involving Cr2O72-?
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer:
Verified
Q66: The following equation, when balanced, involves
Q67: Complete and balance the following equation:
Q68: refer to the following reaction in
Q69: Which of the following isn't true?
A) The
Q70: Write a balanced chemical equation for
Q72: refer to the following incomplete, unbalanced equation
Cr2O72-(aq)
Q73: Write a balanced chemical equation for
Q74: Balance the following redox reaction. Clearly
Q75: Balance the following oxidation-reduction equation.
HI(aq) +
Q76: Balance the following oxidation-reduction equation.
CrO42-(aq) + HSnO2-(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents