Multiple Choice
For the following reaction:
2 NOCl(g) 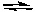 2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
A) The rate of the forward reaction would be exactly equal to the rate of the reverse reaction.
B) Neither the forward nor the reverse reaction would occur because the reaction would be at equilibrium.
C) The rate of the forward reaction would be faster than the reverse reaction.
D) The rate of the reverse reaction would be faster than the forward reaction.
E) There is no relationship between these concentrations and the rate of either the forward or the reverse reaction.
Correct Answer:
Verified
Related Questions

