For the reaction below, 0.0500 moles of ethyl acetate are allowed to react with 0.200 moles of water. At equilibrium it is found that 0.0300 moles of acetic acid have been produced. How many moles of ethyl acetate remain at equilibrium?
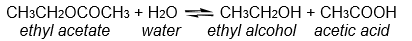
A) 0.0100 moles
B) 0.0300 moles
C) 0.0500 moles
D) 0.170 moles
E) none of these
Correct Answer:
Verified
Q24: If the equilibrium constant for the following
Q25: For the following reaction:
2 NOCl(g) 
Q26: Suppose that the reaction quotient, Qc,
Q27: For the following reaction 2 NO2(g)
Q28: The following reaction was carried out at
Q30: A 0.100 M sample of SO2 reacts
Q31: Calculate the concentrations of Cl2 and ClF3
Q32: For the reaction Q33: What is the relationship between the changes Q34: For the following reaction
2 NOCl(g) ![]()
2 C2H6(g) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents