Ag2SO4(s) is in equilibrium with silver and sulfate ions in solution:
Ag2SO4(s) 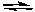 2Ag+(aq) + SO42-(aq)
2Ag+(aq) + SO42-(aq)
Which of the following will not increase the amount of solid Ag2SO4(s) present?
A) boiling off some of the water
B) adding Na2SO4 to the solution
C) adding AgNO3 to the solution
D) adding NaCl to the solution
E) All of the above will increase the amount of Ag2SO4 present.
Correct Answer:
Verified
Q84: A solution is prepared in which the
Q85: If a solution has a Mg2+ ion
Q86: A solution is prepared in which the
Q87: A 50.0 mL solution of 0.015 M
Q88: In which of the following will the
Q89: Which of the following statements is correct?
A)
Q91: What is the concentration of Ag+ (mol/L)
Q92: Approximately how many grams of Ag2CO3 will
Q93: Which of the following changes will
Q94: A solution is 0.10 M in Ca(NO3)2.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents