For the reaction aA (aq) + bB (s) cC (l) + dD (g) ,all of the statements below are TRUE,except:
A) 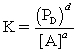
B) concentrations of solutes are expressed in moles per liter.
C) concentrations of gases are expressed in bars.
D) concentrations of pure solids,pure liquids and solvents are omitted because they are negligible.
E) quantities in the equilibrium expression are the ratio of the concentration of the species to the concentration in its standard state.
Correct Answer:
Verified
Q1: When the direction for a reaction is
Q2: In which solution is solubility of silver
Q4: Nickel forms three complexes with hydroxide,NiOH+ (aq),Ni(OH)2
Q5: The molar solubility of a saturated
Q6: All of the following are strong acids,except:
A)HCl.
B)HNO3.
C)HBr.
D)HClO4.
E)HF.
Q7: A student attempts to separate Cl− and
Q8: For a reaction with a Ho
Q9: For the reaction HCN (aq)+ H2O
Q10: The pH of a solution is 9.65.The
Q11: Which of the following is INCORRECT for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents