Consider the following images.The reactions occurring in each are shown in the choices. 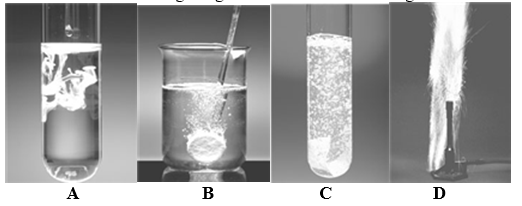
Which of the following is an example of an electron transfer reaction?
A) Pb(NO3) 2(aq) + Na2SO4(aq)  2 NaNO3(aq) + PbSO4(s)
2 NaNO3(aq) + PbSO4(s)
B) H2CO3(aq)  H2O(l) + CO2(g)
H2O(l) + CO2(g)
C) Ca(s) + 2HCl(aq)  CaCl2(aq) + H2(g)
CaCl2(aq) + H2(g)
D) Zn(s) + Cu2+(aq)  Zn2+(aq) + Cu(s)
Zn2+(aq) + Cu(s)
E) Both c and d are electron transfer reactions.
Correct Answer:
Verified
Q7: Consider the following beakers.In the beaker on
Q8: What is the oxidation number of sulfur
Q9: What is the oxidation number of titanium
Q10: Reduction can be defined as...
A)a increase in
Q11: Which of the following is a reduction
Q13: Which among the following are the parts
Q14: How many electrons are transferred in the
Q15: Which of the following statements is correct
Q16: Which of the following is the half-reaction
Q17: What is the balanced redox equation that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents