Which of the following conforms to the Arrhenius definition of an acid?
A) H2O( 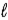 )
)
B) HBrO3(aq)
C) HCO3-(aq)
D) Al3+(aq)
E) Mg(OH) 2(s)
Correct Answer:
Verified
Q6: What is the conjugate acid of HPO42-(aq)?
A)H3PO4(aq)
B)HPO42-(aq)
C)H2PO4-(aq)
D)PO43-(aq)
E)H3O+(aq)
Q7: Which of the following properties is traditionally
Q8: What is a Brønsted-Lowry base?
A)An electron pair
Q9: Which pair below cannot have a Brønsted-Lowry
Q10: Identify the conjugate acid base pairs in
Q12: Which of the following is not capable
Q13: According to Arrhenius theory,what is an acid?
A)A
Q14: Which of the following is a characteristic
Q15: Which of these statements is true?
A)All Brønsted-Lowry
Q16: Consider the following image which depicts the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents