A substance has a melting point of 20°C and a heat of fusion of 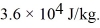
The boiling point is 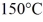
And the heat of vaporization is 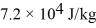
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is given up by 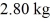
Of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
A) 400 kJ
B) 200 kJ
C) 300 kJ
D) 440 kJ
E) 640 kJ
Correct Answer:
Verified
Q29: A 35-g block of ice at -14°C
Q30: A 44.0-g block of ice at -15.0°C
Q31: A substance has a melting point of
Q32: A 45.0-kg sample of ice is at
Q33: A 400-g block of iron at 400°C
Q35: If you add 700 kJ of heat
Q36: Solar houses use a variety of energy
Q37: The melting point of aluminum is 660°C,its
Q38: If you add 1.33 MJ of heat
Q39: Heat is added to a 3.0 kg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents