Multiple Choice
Which equilibrium constant represents a reaction that favors the reactants to the greatest extent?
A) 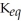 = 100
= 100
B) 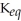 = 1.0 ×
= 1.0 × 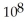
C) 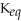 = 1.0 ×
= 1.0 × 
D) 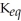 = 1.0 ×
= 1.0 × 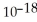
E) not enough information
Correct Answer:
Verified
Related Questions
Q61: For the reaction Q62: For the reaction Q63: For the reaction Q64: For the reaction Q65: Identify the equation for which Q67: For the reaction 2 H2O (l)⇌ 2H2 Q68: For the reaction Q69: For the reaction LiOH (s)⇌ Li+ (aq)+ Q70: Which equilibrium constant represents a reaction that Q71: For the reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

![]()
![]()

