The complete combustion of 1.47 g of methanol produces 29.3 kJ of heat. Determine the DH° for the reaction and its sign. 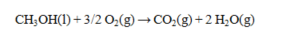
A) -938 kJ
B) -638 kJ
C) -1.35 kJ
D) +638 kJ
E) +938 kJ
Correct Answer:
Verified
Q27: Which process is exothermic?
A) freezing rain drops
B)
Q28: What is the enthalpy change when 22.5
Q29: A bomb calorimeter has a heat capacity
Q30: How much heat is required to melt
Q31: Determine if each of the four processes
Q33: When does an endothermic reaction occur?
A) when
Q34: Which term refers to a quantity of
Q35: Based on the following thermochemical equation, which
Q36: A sample of water containing 2.00 moles
Q37: What is the enthalpy change when 175
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents