Determine the heat of reaction for the process 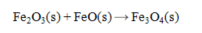
using the information given below:
A) -1074.0 kJ
B) -22.0 kJ
C) 22.2 kJ
D) 249.8 kJ
E) 1074.0 kJ
Correct Answer:
Verified
Q18: Which of the following is not an
Q19: Which of the following is an example
Q20: What is the molar heat capacity of
Q21: Based on the following thermochemical equation below,
Q22: The temperature of 3.50 kg of water
Q24: What is the enthalpy change for the
Q25: A 0.100 mole sample of CH4 reacts
Q26: How much energy is required to melt
Q27: Which process is exothermic?
A) freezing rain drops
B)
Q28: What is the enthalpy change when 22.5
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents