The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.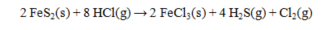
A) -881.4 kJ
B) -811.8 kJ
C) -149.6 kJ
D) +149.6 kJ
E) +213.4 kJ
Correct Answer:
Verified
Q43: The quantity of energy required to increase
Q44: Match the following:
Q45: Enthalpy change is equal to heat transfer
Q46: The standard enthalpies of formation for several
Q47: How much energy in kilojoules is required
Q49: The standard enthalpies of formation for several
Q50: In an endothermic reaction, heat is transferred
Q51: The standard enthalpies of formation for several
Q52: Energy of motion is called _.
Q53: The standard enthalpies of formation for several
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents