Use the data at 25 C given below to calculate the value of DG rxn for the reaction shown when it takes place at 120 C. 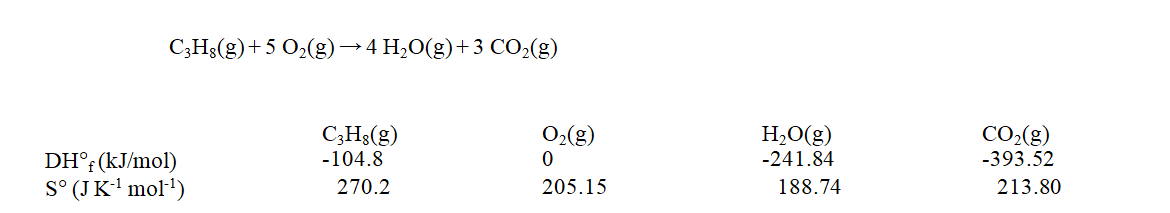
A) -2004 kJ
B) -2046 kJ
C) -2055 kJ
D) -2073 kJ
E) -2083 kJ
Correct Answer:
Verified
Q21: A reaction cannot change between being
Q22: A certain reaction has DH
Q23: At constant T and P, in which
Q24: Calculate the value of DS
Q25: At constant T and P, in which
Q27: According to the Second Law of Thermodynamics,
A)
Q28: At constant T and P, in which
Q29: Which statement is not correct?
A) the
Q30: A reaction is exothermic and has
Q31: Use the data given to calculate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents