Recovering aluminum directly from its ore, which is primarily aluminum oxide, involves the following reaction, for which thermodynamic data is tabulated below: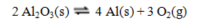

a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q43: Which statement correctly describes the meaning
Q44: If a chemical reaction is at
Q45: Use the data given to calculate
Q46: Use the data given to calculate
Q47: Which set of conditions describes a reaction
Q49: Since no chemical process is 100% efficient,
Q50: Which set of conditions describes a reaction
Q51: What is the value of the
Q52: The value of DG
Q53: A reaction is product-favored when
A) K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents