Consider the Reaction
for Which
C When the Reaction Has Come to Equilibrium, the Concentration
Consider the reaction
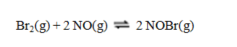 for which
for which
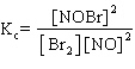 .
.
A sample of pure NOBr is isolated at low temperature. It is placed in a flask at a concentration of 0.200 M and warmed up to 50 C. When the reaction has come to equilibrium, the concentration of NOBr is 0.176 M. What is the value of Kc at 50 C for this reaction?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Consider the reaction Q47: Which statement concerning product-favored reactions is not Q48: Which of the following is true for Q49: Consider the statement, "At equilibrium, a reaction Q50: In predicting which side of an equilibrium Q52: For a particular chemical reaction, which of Q53: Considering only the probability factor in the Q54: Consider the reaction Q55: As the temperature is increased, which kinds Q56: If the value of Kc for a
![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents