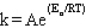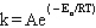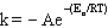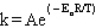Related Questions
Q20: The rate of a chemical reaction may
Q21: The decomposition of N2O(g) to nitrogen
Q22: In a bimolecular reaction, which term can
Q23: A certain reaction is studied at room
Q24: From the stoichiometry of the reaction
Q26: The half-life for the first-order conversion of
Q27: The species representing the combination of atoms
Q28: For a first-order reaction, which expression describes
Q29: A reaction displays zero-order kinetics for its
Q30: The frequency factor in the Arrhenius equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents