In the reaction, HSO4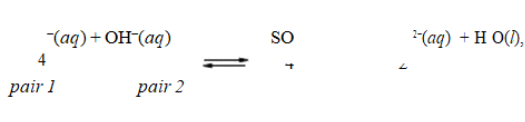 , the conjugate acid-base pairs are
, the conjugate acid-base pairs are
A) HSO − and SO 2−; H O and OH−.4 4
2 
B) HSO4− and H3O+; SO42 − and OH−.
C) HSO4− and OH−; SO42 − and H2O.
D) HSO4− and H2O; OH − and SO42−.
E) HSO4− and OH−; SO42 − and H3O+.
Correct Answer:
Verified
Q36: What is the [H3O+] for a solution
Q38: What is the pH of a 0.050
Q42: What is the pH of a solution
Q43: The pH of a Ba(OH)2 solution is
Q49: What is the conjugate base of HSO4−
Q51: Identify the conjugate base of HPO42− in
Q77: Automobile batteries use 3.0 M H2SO4 as
Q110: During a titration, it is found that
Q114: What volume of a 0.442 M NaOH
Q117: What volume of a 0.452 M NaOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents