Suppose white atoms (W) and black atoms (B) react according to the following balanced chemical equation. 2W + B → BW2
If the initial reaction mixture is prepared as follows, what will the final reaction mixture be once the reaction is complete? 
A) 
B) 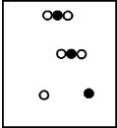
C) 
D) 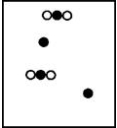
E) 
Correct Answer:
Verified
Q44: When a chemical equation is balanced, it
Q44: Balance the following equation: Ca3(PO4)2(s) + SiO2(s)
Q48: If aqueous solutions of Ba(OH)2 and HNO3
Q51: What is the coefficient of H2O when
Q51: Balance the following equation: C8H18O3(l) + O2(g)
Q52: What is the coefficient of H2SO4 when
Q53: What is the coefficient of O2 when
Q55: What is the coefficient of H2O when
Q55: Once the following equation is balanced with
Q58: Once the following equation is balanced with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents