What is wrong with this Lewis structure? 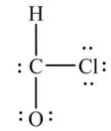
A) There are too many electrons.
B) There are too few electrons.
C) The O atom does not have an octet.
D) The C atom does not have an octet.
E) There is nothing wrong with this Lewis structure.
Correct Answer:
Verified
Q8: Using the VSEPR model, predict the molecular
Q10: According to the VSEPR model, the predicted
Q12: In which one of the following is
Q13: The Lewis structure for CS2 is:
A)
Q19: Which of these choices is the best
Q20: Which pair of Lewis structures is not
Q24: What is the molecular geometry of HOF
Q26: The Lewis structure for a chlorate ion,ClO3-,should
Q59: The number of resonance structures for the
Q78: The total number of bonding electrons in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents