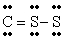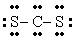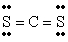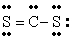Related Questions
Q24: Which of the elements listed below has
Q31: Which of the bonds below would have
Q34: Which of the atoms listed below is
Q42: The number of lone electron pairs in
Q49: The number of lone electron pairs in
Q68: The formal charge on the bromine atom
Q69: Assuming the octet rule is obeyed, how
Q72: Assuming the octet rule is obeyed, how
Q78: The total number of bonding electrons in
Q79: Assuming the octet rule is obeyed, how
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents