Multiple Choice
The number of pi bonds in the molecule below is 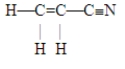
A) 1.
B) 2.
C) 3.
D) 5.
E) 9.
Correct Answer:
Verified
Related Questions
Q61: What is the hybridization on the central
Q72: The hybridization of the central nitrogen atom
Q73: If a triatomic molecule is linear, then
Q75: Indicate the type of hybrid orbitals used
Q80: In which one of the following molecules
Q83: In which of the following would the
Q86: Which of the following species have the
Q89: The number of pi bonds in the
Q90: Consider the species O2-, O2, and O2+.
Q98: Use VSEPR theory to explain why the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents