Based on the phase diagram shown below, which is more dense: the liquid phase or the solid phase? 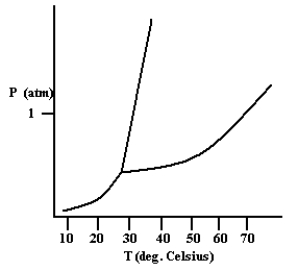
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q101: Of the given pair of compounds, which
Q103: Copper crystallizes in a face-centered cubic unit
Q120: Of the pair of compounds given, which
Q122: Boron nitride, BN3, melts at approximately at
Q123: Suppose the atoms in a two-dimensional crystal
Q125: Calculate the amount of enthalpy required
Q129: The fact that the density of ice
Q132: Magnesium oxide, MgO, melts at 2,800°C and
Q144: Ethanol (C2H5 - OH) will have a
Q149: Given that the heat of vaporization of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents