Calculate ΔS° for the formation of one mole of solid sodium bromide from the elements at 25°C. 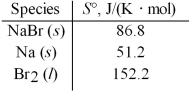
A) -116.7 J/K
B) -81.2 J/K
C) -40.5 J/K
D) 86.8 J/K
Correct Answer:
Verified
Q1: Predict the sign of ΔS for each
Q19: The Boltzmann formula is S = k
Q28: What is the sign of ΔS for
Q30: Under which of the following conditions would
Q31: Which of the following gas molecules has
Q32: Which provides the greatest increase in entropy?
A)H2O
Q35: Which substance has the highest standard molar
Q36: Which has the highest entropy in each
Q37: Which has the highest standard molar entropy
Q38: Calculate ΔS° for the following reaction.
N2(g)+ 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents