Consider an initial mixture of CH4 and O2 represented in the container below: 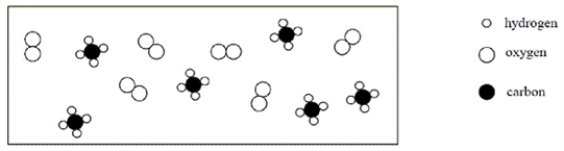 Given the reaction CH4 + 2O2 → CO2 + 2H2O,which of the following represents a stoichiometric picture of the container after the reaction has gone to completion?
Given the reaction CH4 + 2O2 → CO2 + 2H2O,which of the following represents a stoichiometric picture of the container after the reaction has gone to completion?
A) 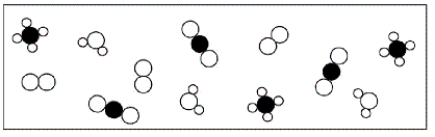
B) 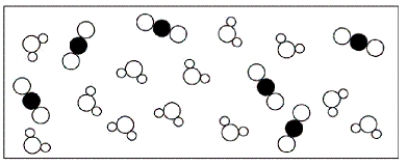
C) 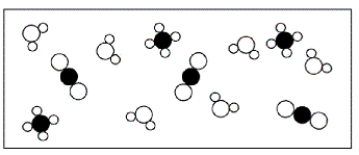
D) 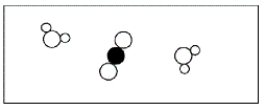
E) none of the above
Correct Answer:
Verified
Q96: A given hydrocarbon is burned in the
Q97: A compound is composed of only C
Q98: An organic compound has a molar mass
Q99: A sample containing 0.400 mol of a
Q100: A certain compound has a molar mass
Q102: A compound contains 43.84 % carbon atoms,3.65%
Q103: The products of the combustion of acetone
Q104: One step in the isolation of pure
Q105: Ammonia,NH3,and oxygen can be reacted together in
Q106: Complete combustion of a 0.40-mol sample of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents