Given the following data,calculate the standard enthalpy of reaction for the conversion of buckminsterfullerene (C60) into diamond: 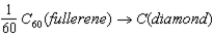 C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
60C(graphite) → C60(fullerene) ; ΔH° = +2193 kJ
A) -35 kJ
B) 35 kJ
C) −2191 kJ
D) 2191 kJ
E) 38 kJ
Correct Answer:
Verified
Q95: For which of the following equations is
Q96: Which of the following reactions corresponds to
Q97: Given the following thermochemical data at 25°C
Q98: What is the standard enthalpy of formation
Q99: Which substance has a standard enthalpy of
Q101: What is the standard enthalpy of formation
Q102: What is the standard enthalpy of formation
Q103: Which of the following is not a
Q104: What is the standard enthalpy of formation
Q105: A 70.4-L sample of a gaseous hydrocarbon,measured
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents