Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g) 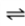 O2(aq)
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
A) The amount of O2 dissolved in the liquid decreases.
B) The amount of O2 dissolved in the liquid increases.
C) The amount of O2 dissolved in the liquid does not change.
D) Not enough information is provided to answer the question.
E) Either A or B.
Correct Answer:
Verified
Q5: The solubility of 1-pentanol in water is
Q6: Which of the following is a correct
Q7: Which of the following statements best describes
Q8: Which of the following compounds is least
Q9: According to the National Institute of Standards
Q11: Consider the following gas-liquid equilibrium for an
Q12: Which of the following correctly states the
Q13: Which of the following sets of conditions
Q14: At a particular temperature the solubility of
Q15: Which of the following pure liquids is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents