Multiple Choice
Consider the following equilibrium:
4NH3(g) + 3O2(g) 
2N2(g) + 6H2O(g)
Suppose 0.30 mol of NH3 and 0.40 mol of oxygen are added to a 5.0-L container.If x mol of water is present at equilibrium,how many moles of ammonia will remain at equilibrium?
A) 0.30 - 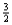 x
x
B) 0.30 - x
C) 0.30 - 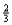 x
x
D) 0.40 - 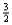 x
x
E) 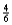 x
x
Correct Answer:
Verified
Related Questions
Q40: Carbon tetrachloride may react with oxygen to
Q41: For the reaction 2H2S(g) Q42: Consider the following equilibrium: Q43: Consider the following equilibrium:![]()
C2H6(g)+ C5H12(g) 
2NO(g)+ 3F2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents