For the reaction:
3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g)
Write the expression for Kp.
A) 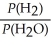
B) 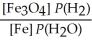
C) 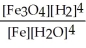
D) 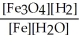
E) 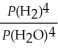
Correct Answer:
Verified
Q58: Consider the following equation:
N2O4(g)⇌ 2 NO2(g),Kc =
Q59: What is the equilibrium constant expression for:
4
Q60: Write the equilibrium constant expression for the
Q61: Write the equilibrium expression Kc for the
Q62: For a reaction,the reaction quotient,Qc > Kc
Q64: For the reaction 2 NO2(g)⇌ N2O4(g)Kp equals
Q65: Two moles of NH3 are initially present
Q66: For the reaction: 3 Fe(s)+ 4 H2O(g)⇌
Q67: What is the relationship between Kp and
Q68: For the reaction: 3 Fe(s)+ 4 H2O(g)⇌
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents