Using normal bonding requirements for atoms, the following correctly shows the bonding in a compound with the formula C2H3N. 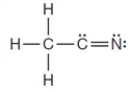
Correct Answer:
Verified
Q6: Examine the following image showing a "subatomic"
Q7: The Lewis structure for Cl2O could be
Q8: Aluminum and oxygen form a compound with
Q9: The formula that corresponds to the IUPAC
Q11: Phosphorus must gain three electrons to obey
Q12: A possible bonding pattern for an element
Q13: Sodium loses an electron in forming an
Q14: Based on the number of valence electrons,
Q15: Sodium combines with oxygen to form Na2O.
Q63: Metals gain electrons to become ions in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents