The isomerization of cyclopropane to form propene is a first-order reaction. 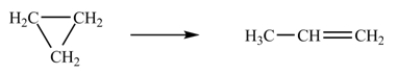 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
A) 3.4 × 10-2 min
B) 2.5 min
C) 23 min
D) 29 min
E) 230 min
Correct Answer:
Verified
Q24: A reaction was experimentally determined to follow
Q28: A city's water supply is contaminated with
Q32: Appropriate units for a second-order rate constant
Q34: At 25°C the rate constant for the
Q36: The following initial rate data apply
Q36: A city's water supply is contaminated with
Q39: A certain first-order reaction A
Q41: The thermal decomposition of acetaldehyde, CH3CHO
Q42: A certain reaction A
Q43: The graphs below all refer to the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents