A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 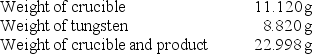 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
Correct Answer:
Verified
Q123: A chemistry student determined the empirical formula
Q131: Balance the following chemical equation:
H2 +
Q145: What percent by mass of oxygen is
Q146: If 0.66 mole of a substance has
Q147: Calculate the volume of 0.15 mole of
Q150: Calculate the percent composition by mass of
Q151: Phosgene, a poisonous gas used during WWI,
Q153: A sample of unknown ore was analyzed
Q160: A 0.600 g sample of a compound
Q165: Balance the following chemical equation:
C3H6O +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents