In the reaction H2CO3 + H2O 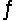 HCO3- + H3O+, the Brønsted acids are
HCO3- + H3O+, the Brønsted acids are
A) H2CO3 and H2O.
B) HCO3- and H2CO3.
C) H2O and H3O+.
D) H3O+ and H2CO3.
E) H2O and HCO3-.
Correct Answer:
Verified
Q8: What is the pH of an aqueous
Q9: Identify the conjugate base of HSO4 -
Q9: The OH- concentration in a 2.5 *
Q10: Identify the conjugate base of HPO42- in
Q12: Which is not a characteristic property of
Q14: What is the concentration of H+ in
Q14: Identify the conjugate acid of CO32- in
Q15: Identify the conjugate base of HClO3 in
Q16: Which is the formula for the hydronium
Q18: Identify the conjugate base of CH3COOH in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents